

In 1896 Goldstein noticed that when a discharge occurred in a
tube of low-pressure gas rays appeared to be produced originating from the anode. These
he called positive rays, sometimes known as canal rays.
The rays originated within
the gas itself, none being produced if the tube were totally evacuated.
It was
realised that these positive rays were composed of particles, and that these particles were
positive ions of the gas in the tube. The properties of the positive rays were first investigated
by Thomson in 1906, using the apparatus shown in Figure 1(a).
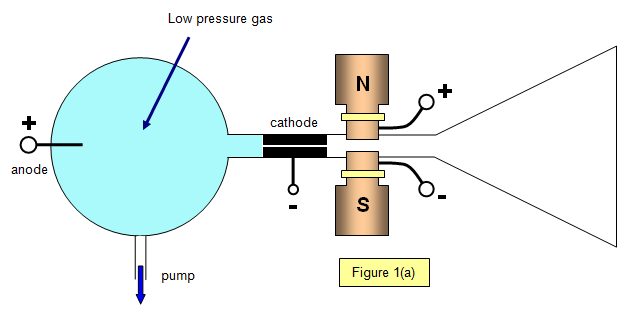
A discharge occurs in the low-pressure gas in the bulb at the left-hand end of the apparatus, producing positive rays that pass through the anode and into the right-hand end of the tube. Here they go through electric and magnetic fields that act in the Y-direction; the electric and magnetic forces on the particles are therefore at right angles to each other.

When the particles strike the fluorescent screen a parabola (Figure 1(b))
is produced by particles having different velocities, those with the highest velocities having
originated closest to the anode (since an ion with zero velocity near the anode will have a
longer distance over which to accelerate before it reaches the cathode if it makes no
collisions on the way).
Two parabolae are shown made by
particles with different masses m1 and m2 (m1>m2).
The positive rays showed the
following properties:
(a) they could be deflected by electric and magnetic fields;
(b)
they showed a spectrum of velocities;
(c) they were dependent on the gas in the
tube;
(d) they could cause ionisation;
(e) they caused fluorescence and affected
photographic plates.
It was assumed that the particles were gaseous ions of mass
m and charge q.
J.J.Thomson found that the value of q/m for the particles in the
positive rays with hydrogen in the tube was of the order of 108 Ckg-1. This must mean that
the particle that makes up positive rays has either a smaller charge than that of the electron
or a much greater mass, and it is the latter that has been accepted.